Caustic soda, also known as sodium hydroxide (NaOH)
Caustic soda, also known as sodium hydroxide (NaOH), is an essential chemical compound with a wide range of applications. Let’s delve into its preparation, properties, and uses:
- Preparation of Caustic Soda:
- There are several methods to produce sodium hydroxide:
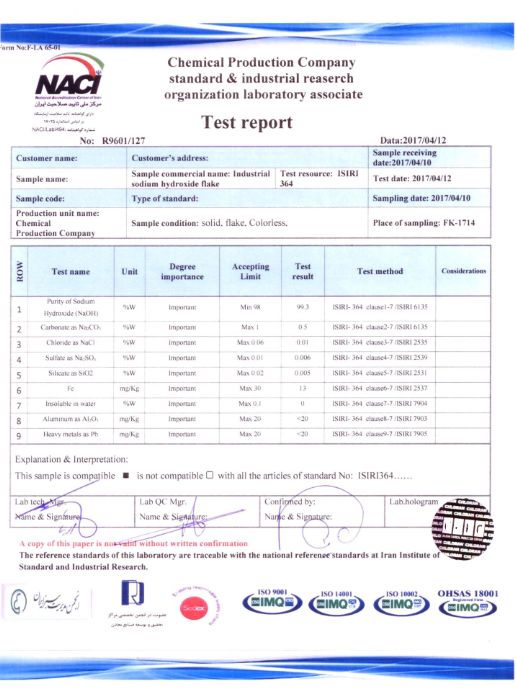
- Castner-Kellner Process: In this method, electrolysis of brine solution (sodium chloride, NaCl) is performed to obtain sodium hydroxide.
- The Castner-Kellner cell consists of a rectangular steel tank lined with ebonite. Titanium acts as the anode, and a layer of mercury at the tank’s bottom serves as the cathode.
- Ionization of brine solution occurs:
2\text{NaCl} \rightarrow 2\text{Na}^+ + 2\text{Cl}^-2NaCl→2Na++2Cl−
- Sodium ions deposit at the mercury cathode, forming a sodium amalgam. Chlorine ions move toward the anode and exit the cell.
- Reaction at the anode:
2\text{Cl}^- \rightarrow \text{Cl}_2 + 2\text{e}^-2Cl−→Cl2+2e−
- Reaction at the cathode:
2\text{Na}^+ + 2\text{e}^- \rightarrow 2\text{Na}2Na++2e−→2Na
- The amalgam is then transferred to another chamber (denuder) and treated with water to obtain a sodium hydroxide solution. Upon evaporation, solid caustic soda is formed.
- Other methods include the Nelson Diaphragm cell and Loewig’s process.
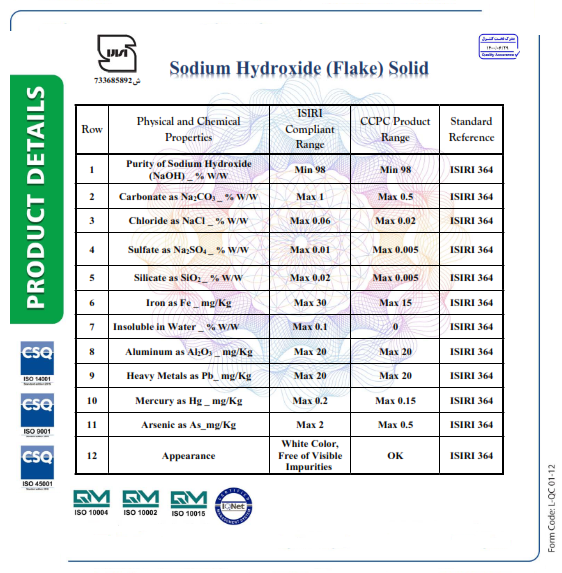
- Properties of Caustic Soda:
- It is a white solid with a melting point of 591K.
- Sodium hydroxide is stable, bitter, and has a soapy feel.
- Highly soluble in water and moderately soluble in alcohol.
- Strongly alkaline in nature.
- Caustic Soda Uses:
- Cleansing Agent: Caustic soda is used for cleaning purposes.
- Manufacturing of Washing Soda: It plays a role in the production of washing soda (sodium carbonate).
- Laboratory Reagent: Sometimes, sodium hydroxide is used as a reagent in laboratories.
- Preparation of Soda Lime: Used in the preparation of soda lime, which absorbs carbon dioxide.
- Aluminium Extraction: Caustic soda purifies bauxite during the extraction of aluminum.
Remember that caustic soda is highly corrosive to animal and plant tissues, so proper handling and safety precautions are essential when working with it.